Clunky versioning slowing down your compliance?
ComplianceQuest often frustrates users with its complex audit trails, limited integrations, and confusing version control options that feel anything but intuitive.
This can create extra headaches for quality teams trying to stay audit-ready while juggling constant changes and mounting compliance challenges.
Every minute spent navigating complex menus or managing broken workflows means lost productivity and growing audit anxiety. That delay in documentation accuracy can snowball into bigger compliance risks, unhappy teams, and missed deadlines. If your software is slowing audits and leaving your processes fragmented, the real cost is daily disruption that only grows over time.
Thankfully, there are strong options out there that offer smoother audit trails and effortless control over your compliance documents.
In this article, I’ll introduce the best best ComplianceQuest alternatives for document management software in quality control, reviewing tools like FileCenter, SimplerQMS, ETQ, and MasterControl.
With the right switch, you can finally streamline audits, integrations, and team collaboration.
Let’s explore your options.
Quick Summary:
| # | Alternative | Rating | Best For |
|---|---|---|---|
| 1 | FileCenter → | Budget-friendly intuitive control | |
| 2 | SimplerQMS → | Life science teams, fast launch | |
| 3 | Paradigm 3 → | Flexible multi-format document use | |
| 4 | ETQ Reliance → | Teams needing agile workflows | |
| 5 | QT9 QMS → | Regulated users valuing validation |
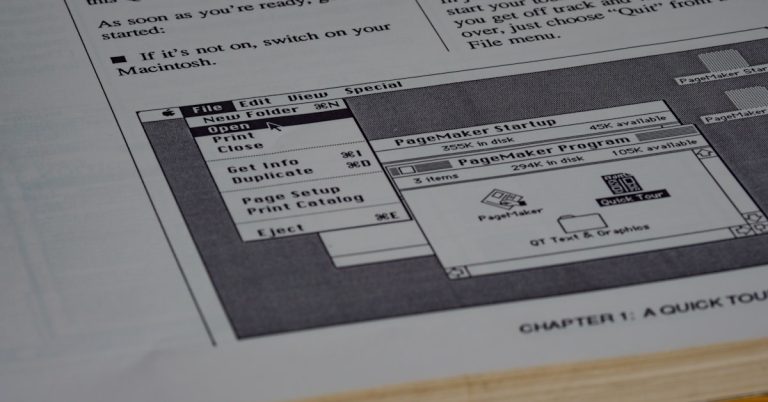
1. FileCenter

Frustrated by ComplianceQuest’s complicated versioning and workflow?
FileCenter offers an intuitive desktop-first solution with Windows-style cabinet-drawer filing, skipping the heavy, complex setups found in enterprise QMS software like ComplianceQuest.
If you’re after simplicity, FileCenter uses familiar folder structures to eliminate confusing database navigation and supports straightforward organization right out of the box.
You want relief without the usual hassle.
Here’s how FileCenter stands out as an alternative to ComplianceQuest, especially if you want reliable quality control without the enterprise bloat.
FileCenter gives you a cabinet-and-drawer filing experience, so team adoption is quick—no long training cycles or confusing configurations. Integrated scanning with OCR pulls your paper workflows straight into the digital archive, using intelligent file naming that speeds up audits and reduces manual work.
Plus, you don’t get locked into endless subscriptions. FileCenter’s one-time desktop license is simple and affordable, so if your team prefers a predictable investment, you can finally avoid those ongoing cloud costs.
The result: document management gets easier and audit trails more efficient.
Key features:
- Cabinet-drawer filing with Windows folder structure so your quality team can start organizing documents instantly, skipping the rigid hierarchy and learning curve you’d expect from ComplianceQuest.
- Integrated scanning and OCR for easy digitization converts paper files into searchable documents while assigning intelligent names, streamlining compliance and cutting down setup time dramatically.
- One-time purchase option for cost savings lets you own your software and plan your budget predictably, instead of dealing with ComplianceQuest’s perpetual subscription expenses.
Verdict: FileCenter is your no-fuss ComplianceQuest alternative for quality control managers who need document management that’s intuitive, quick to deploy, and budget-friendly. With its Windows-like structure and built-in OCR for compliance, it’s a smart fit if you want your team productive within hours, not weeks.
2. SimplerQMS
Tired of ComplianceQuest’s drawn-out implementations?
SimplerQMS offers rapid deployment, with your full quality management system up and running within just 5-6 weeks—no endless configuration headaches or hidden fees in sight.
Where ComplianceQuest frustrates with long deployment timelines and confusing tiered pricing, SimplerQMS’s all-inclusive subscription delivers every QMS module, ongoing validation, training resources, and strong support under one predictable price. This means your quality control team can forget budget surprises and start focusing on compliance from day one.
You skip the drawn-out customization cycle entirely.
Here’s how SimplerQMS changes the equation for quality management.
Instead of wrestling with complex migration steps or workflow disruption, you get everything bundled—QMS modules, validation, and onboarding assistance. Plus, customizable Microsoft Office-based SOP and document templates mean your team doesn’t waste time building forms from scratch. You can use familiar file formats for work instructions and reports right away, making adoption easy if you’re coming from ComplianceQuest.
Additionally, SimplerQMS’s implementation process is specifically designed for life sciences, so compliance is baked in from the start. The transparent pricing includes everything your team will need, with support and validation ongoing—no ambiguous service tiers or surprise costs stalling your transition. Your switch feels intuitive, not intimidating.
You’re set up for audit success much faster.
Key features:
- All-inclusive validated QMS subscription brings together every critical module, validation, and hands-on support in a flat-rate annual plan, eliminating hidden fees or complex tiered pricing.
- Rapid 5-6 week implementation enables your whole team to start managing digital quality processes and audit trails with minimal disruption, unlike long rollouts found in enterprise QMS platforms.
- Ready-to-use Microsoft Office templates simplify consistent document creation for SOPs, work instructions, and reports, so your staff gets started immediately—no deep training on unfamiliar formats required.
Verdict: SimplerQMS stands out as a strong ComplianceQuest alternative for document management in quality control. With full deployment in as little as 5-6 weeks, bundled validation, and familiar templates, life science teams can reduce onboarding headaches and start driving compliance with a single, predictable annual fee.
3. Paradigm 3

Feeling boxed in by ComplianceQuest’s limited document ecosystem?
Paradigm 3 stands out by giving you multi-format support, custom form design, and automated document review schedules—tackling the very issues ComplianceQuest users often face with restrictive compatibility and manual updates.
Unlike the rigid workflows of ComplianceQuest, Paradigm 3 lets you manage every file type you actually use daily—Word, Excel, PDF, or CAD—right in one centralized platform.
Here’s why that difference matters more than you think.
Paradigm 3 makes switching from ComplianceQuest straightforward
When you move to Paradigm 3, you instantly get real document control that flexes to fit your quality process. No more juggling add-ons or converting files—everything from Word policies to CAD diagrams fits in one unified system.
Plus, you can build custom quality forms and adapt workflows instantly to your process—critical if your team’s tired of ComplianceQuest’s out-of-the-box forms that box you in.
Automated review schedules also mean no more missed compliance deadlines or endless chasing—Paradigm 3 does the reminders for you, so your audit preparedness is always on point, not waiting for a manual check.
Your compliance process finally feels under control.
Key features:
- All-in-one document compatibility supports Word, Excel, PDF, CAD so your team can manage every format you need for quality control, not just a handful like in typical QMS software.
- Flexible drag-and-drop form designer adapts to your workflow, letting you create or update quality, safety, and environmental forms without being stuck with rigid templates.
- Automated yearly document review alerts keep your compliance programs up to date, reducing manual admin and late reviews common in less automated alternatives.
Verdict: Paradigm 3 is a compelling ComplianceQuest alternative for quality control document management. Its all-format document compatibility, workflow-tailored forms, and automated compliance reviews give you the flexibility ComplianceQuest often lacks—especially if your industry relies on complex document types or evolving quality processes.
4. ETQ Reliance
Still battling rigid workflows and slow revisions?
ETQ Reliance streamlines your document management with no-code configuration, integrated quality modules, and highly flexible workflow customization right out of the box.
If ComplianceQuest’s limited adaptability has held back your team, you’ll see how ETQ Reliance offers more workflow agility and unified quality management to meet complex compliance requirements, unlike the rigid processes often found in more restrictive systems.
Switching to ETQ Reliance sets your quality documentation free.
Here’s how ETQ Reliance gives you an edge as a ComplianceQuest alternative.
With ETQ Reliance, you solve workflow rigidity immediately, thanks to its no-code and low-code platform. It lets you tailor document reviews and approval flows without IT bottlenecks, so adaptation is in your hands.
Additionally, integrated modules connect document control, audits, and CAPA, keeping your audit trail, corrective actions, and training data all within one space. This means you eliminate data silos that complicate compliance in less connected systems.
Plus, you can configure and deploy process changes without third-party developers. That way, every update to your quality system is easier, and you keep your quality control program both current and compliant with less stress.
The result: your compliance processes move at the speed you want.
Key features:
- Flexible workflow customization with no-code tools lets you adapt your document review and approval workflows as requirements evolve, directly from the platform, without IT delays or outside consultants.
- Integrated quality management modules connect document control, CAPA, audits, and training so your quality data, corrective actions, and compliance evidence stay unified and traceable.
- Low-code application builder for rapid changes empowers your business users to configure and deploy new quality processes quickly, keeping your QMS responsive to new challenges and regulatory shifts.
Verdict: ETQ Reliance stands out as a superior alternative to ComplianceQuest for document management software dedicated to quality control. Its no-code workflow customization, integrated quality modules, and low-code scalability let you address unique compliance needs—making it ideal for teams who need system agility and unified quality processes.
5. QT9 QMS

Is ComplianceQuest’s interface holding your team back?
QT9 QMS gives you an all-in-one, cloud-based system with 25+ built-in modules covering document control, CAPA, training, and risk management—no extra charges or complex add-ons needed.
Unlike ComplianceQuest, you’re not stuck paying for every named user. Concurrent user licensing means you pay only for those logged in at one time, making it more affordable for teams with fluctuating needs.
Making the switch is easier than you might expect.
Here’s how QT9 QMS steps up as a robust ComplianceQuest alternative that eases your daily document management burdens for regulated quality control.
QT9 QMS covers everything you need from version control to corrective actions and risk management, so you’re not scrambling for missing workflow modules. Pre-validated off the shelf, you get IQ, OQ, and PQ out of the box, slashing your validation workload for FDA, ISO, or EU regulations. This means you’ll never get bogged down with never-ending compliance checklists.
Additionally, implementation typically takes less than 30 days—miles ahead of ComplianceQuest’s longer onboarding. The integrated modules all sync together, so you won’t waste hours integrating or troubleshooting separate tools.
User-driven design, real-time collaboration, and cost-effective access make for a less stressful way to meet quality compliance.
Key features:
- 25+ pre-integrated quality modules included at launch covering document control, CAPA, and training management, letting you skip the hassle and cost of piecemeal add-ons required by other systems.
- Concurrent user licensing for cost effectiveness so your team can share licenses based on who’s active, instead of paying for every single named user all year.
- Pre-validated software with built-in FDA/ISO protocols means you skip lengthy validation projects and can achieve audit readiness far quicker compared to more labor-intensive setups.
Verdict: QT9 QMS is a compelling alternative to ComplianceQuest if you handle quality control for regulated industries and want breadth without hidden costs. Its out-of-the-box validation and 30-day average go-live mean you can accelerate compliance, reduce audit risks, and streamline your entire document lifecycle right away.
6. PSC TruQMS
Tired of ComplianceQuest’s complex document workflows?
PSC TruQMS provides a purpose-built quality management system with deep life sciences expertise, offering configurable document workflows and stronger regulatory coverage than what you usually get with ComplianceQuest.
With PSC TruQMS, you’re not limited to rigid processes—document workflows can match your team’s structure and approval needs, which helps make quality control less tedious and more reliable.
Here’s how PSC TruQMS meets those demands.
You get a solution tailored for strict regulatory standards in life sciences, which means you don’t have to worry about gaps in compliance if you’re leaving ComplianceQuest behind.
Unlike generic platforms, PSC TruQMS lets you configure your document creation, collaboration, and approval process around unique operational or regulatory requirements. If you’ve hit a wall with ComplianceQuest’s one-size-fits-all structure, this flexibility lightens your team’s day-to-day workload.
Additionally, integrated audit management centralizes everything for audit prep—finding, tracking, and resolving findings is far less stressful when it’s all connected in a single place. Features like this work together to boost audit readiness and give you more confidence switching from ComplianceQuest.
Everything’s designed to minimize frustration.
Key features:
- Purpose-built for life science compliance with FDA 21 CFR Part 11 and Part 820 support, offering far more targeted regulatory controls than standard QMS systems like ComplianceQuest.
- Fully configurable document workflows and hierarchies, letting you align creation, review, and approval processes with your specific operational and regulatory needs—no forced templates or rigid models.
- Integrated audit management centralizes documentation and audit trail tracking, giving you clearer audit findings and reducing your team’s stress as deadlines approach.
Verdict: PSC TruQMS is a powerful alternative to ComplianceQuest if you’re in life sciences and serious about compliance. Its tailored document management, configurable workflows, and centralized audit system provide greater control. For regulated teams, it’s everything ComplianceQuest should be—built for your needs, not generic checkboxes.
7. Orcanos

Tired of clunky document controls in ComplianceQuest?
Orcanos brings together product design and quality management in one platform, connecting all your ALM and QMS activities so nothing slips through the cracks.
Unlike ComplianceQuest, Orcanos makes sure your requirements, design outputs, and manufacturing records always link together, giving you real traceability and far less manual data wrangling during audits.
Plus, Orcanos is built for intuitive handling of complex medical and quality workflows.
Let’s look closer at what switching to Orcanos means.
You get a unified alternative for your quality control headaches with Orcanos, especially if ComplianceQuest’s limited integrations and sluggish audit trails keep setting you back.
Here’s how: Orcanos gives your team integrated design control (ALM), so your product development data never gets siloed from your quality history. This tight linkage from specifications to release saves you hours normally lost reconciling changes for compliance documentation. It’s purpose-built for MedTech and life sciences, where audit-readiness is non-negotiable.
Additionally, Orcanos delivers secure electronic signature approvals that meet 21 CFR Part 11, shaving days off review cycles you used to spend tracking down approvals in less integrated tools. The flexible QMS lets you customize every workflow, so you’re not boxed in by out-of-the-box limitations or generic document templates.
Orcanos helps you keep your quality processes moving.
Key features:
- Integrated design control with built-in traceability unites product requirements, development, and manufacturing data in one place, minimizing disconnected silos and reducing manual effort for audit readiness.
- Electronic signature approvals for compliance offer legally compliant, secure digital sign-off on every document, streamlining approval cycles beyond ComplianceQuest’s less integrated or hybrid signing approaches.
- Customizable quality management workflows enable you to build processes tailored to your specific needs, delivering more flexibility and adaptability vs. rigid, preconfigured alternatives.
Verdict: Orcanos stands out as an alternative to ComplianceQuest for document management in quality control. Its unified ALM-QMS saves you weeks on documentation and brings proven value for regulated MedTech product launches, with customizable workflows and secure, audit-ready approvals all in one place.
8. Interfacing Enterprise Process Center
Audit trails with ComplianceQuest feeling too complex lately?
Interfacing Enterprise Process Center tackles this with embedded process modeling, real document workflow automation, and robust records management that many ComplianceQuest users wish they had.
What sets it apart is how it links your quality documents directly to real operational processes for better context and workflow control, instead of leaving files stranded in separate modules.
This means your compliance isn’t siloed anymore.
Interfacing Enterprise Process Center solves document chaos by tying procedures, records, and policies right back to the actual steps your team follows. You’re not forced into generic templates or clunky document libraries like you might be with ComplianceQuest.
You’ll appreciate the visual designer for mapping workflows and the way you can centralize documents with audit trails in process context for richer QA traceability. This takes the guesswork out of version control, so auditors and team members always have the right information at their fingertips.
Plus, built-in compliance modules let you align your documentation with regulatory and risk frameworks, making audit readiness less of a scramble. With process automation and centralized records, your team can cut out duplicate work and avoid the manual tracking that often plagues ComplianceQuest users.
The result is more control, cleaner audits, and happier teams.
Key features:
- Visual process modeling with workflow automation lets you map and automate document-related processes, driving better efficiency and oversight versus static document storage found in ComplianceQuest.
- Integrated document and records management connects files directly to business processes, unifying version control and access for stronger quality assurance and traceability.
- Comprehensive GRC alignment brings quality documents, regulations, and risk frameworks under one platform, ensuring real audit readiness and streamlined compliance management without system silos.
Verdict: Interfacing Enterprise Process Center is your alternative to ComplianceQuest if you want document management fully embedded within quality process flows. Its process-centric approach, workflow automation, and built-in GRC functions provide more context and control for all your quality compliance documentation needs.
9. DocTract
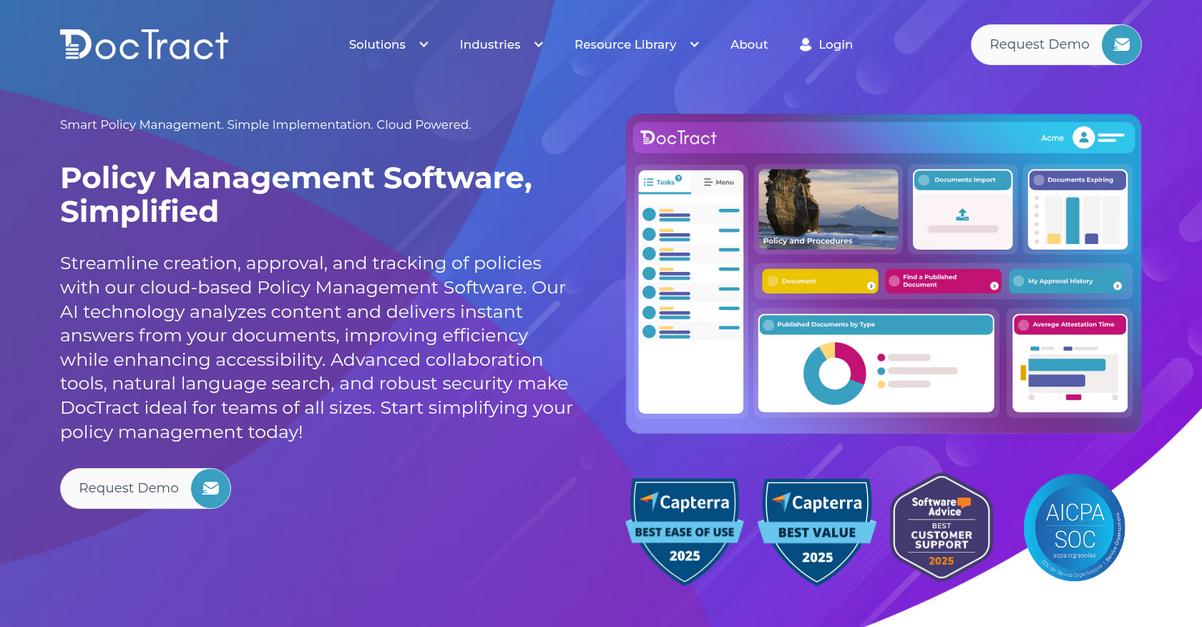
Is ComplianceQuest holding your quality processes back?
DocTract steps in with automated review cycles, real-time version comparison, and an intuitive policy management interface—precisely where ComplianceQuest can make things harder for your team.
Unlike ComplianceQuest’s complex workflows, DocTract offers automated document review and approval with intuitive navigation to cut manual effort and reduce delays for quality managers.
Your compliance headaches have a practical fix.
DocTract’s smart workflows immediately take the stress out of policy and document management. This means you get a clean, user-friendly dashboard for tracking documents across the whole lifecycle without the painful configuration hurdles you might associate with ComplianceQuest.
Here’s how switching to DocTract pays off.
Automated review cycles keep every policy up to date, so you spend less time chasing approvals. You won’t be left guessing about updates, since real-time version comparison makes every change fully transparent for your audit trail and regulatory needs.
Additionally, DocTract’s intuitive interface and accessible policy editing mean your entire team can adopt new processes quickly. IT bottlenecks shrink and version anxiety fades, so your quality control always stays ahead of audits.
The result is clarity and speed for your team.
Key features:
- Automated review and approval cycles cut manual workload and eliminate the appointment-chasing and reminders common in traditional QMS approvals, keeping your quality documents and policies always current.
- Real-time version comparison across all documents offers unmatched transparency into policy changes, revealing exactly who changed what—this is far beyond basic version histories you may get elsewhere.
- Intuitive, user-friendly policy management interface empowers non-technical users to create, update, and publish compliance documents, driving faster adoption and less IT dependency than ComplianceQuest’s configuration-heavy approach.
Verdict: If you need an alternative to ComplianceQuest for document management in quality control, DocTract deserves your attention. Quality leads report significant time savings and easier compliance audits—with DocTract’s intelligent automation and user-first interface, your document lifecycle management just gets simpler and more reliable.
10. MasterControl
Tired of ComplianceQuest’s document control slowing you down?
MasterControl tackles key ComplianceQuest frustrations by automating document distribution, routing, and approvals. That means audit trails are easier to track, while version control actually works in real time.
Your team gets fewer manual errors and less confusion with Automated Document Distribution and Approval at every workflow stage. Instead of worrying about missed review steps or bottlenecks, you get tighter control, which steers clear of compliance gaps common with less automated systems.
Ready to see what makes MasterControl the smarter switch?
MasterControl solves these issues by giving you an enterprise-grade QMS that’s built for regulated, quality-controlled environments—especially if you’re in pharma or medical devices and need validation-ready document handling.
The platform’s robust audit trail delivers secure, time-stamped proof for every document change, making compliance audits far simpler than with ComplianceQuest’s basic tracking. Whenever a document is updated, linked training management auto-triggers retraining, ensuring staff are always up to date on the latest SOPs.
Additionally, MasterControl integrates all your core quality processes—from document management to training—in one system. This means your audit-readiness, regulatory submissions, and team onboarding improve, without the migration headaches and workflow disruption you fear from switching.
You get control and real efficiency at every step.
Key features:
- Automated routing, review and approval flows accelerate document changes and feedback loops, reducing manual errors and delays that can plague less-automated systems like ComplianceQuest.
- Robust, secure audit trail for every action provides the transparency, traceability, and compliance proof you need for regulatory audits, exceeding what most alternatives offer today.
- Integrated training management linked to documentation ensures employees stay competent and retrained automatically with each document revision, minimizing the administrative burden during SOP updates.
Verdict: MasterControl stands out as an alternative to ComplianceQuest for document management and quality control. Its enterprise-grade automation, 21 CFR Part 11-ready audit trails, and integrated training can help you reduce compliance costs and accelerate product launches. Regulated firms report up to 30% shorter audit prep times.
Conclusion
Tired of clunky versioning and audit trail headaches?
ComplianceQuest often leaves you stuck with confusing workflows, limited integrations, and audit management that feels far from seamless.
That’s exactly why your decision to switch is a smart one—escaping daily inefficiencies means reclaiming your team’s momentum and giving your compliance efforts the upgrade they deserve.
Let’s focus on a better solution.
I’ve found FileCenter to be the clear winner if you want to leave ComplianceQuest’s frustrations behind. It streamlines document control, slashes workflow confusion, and gives you simplicity without sacrificing functionality.
FileCenter stands out because it’s quick to adopt and built for users who need straightforward document organization—no bloated QMS complexity required. It’s the ComplianceQuest alternative that lets your quality team organize, retrieve, and share documents with zero hassle.
Go ahead and start a free trial of FileCenter—you deserve document management that just works.
Enjoy smoother audits and easier compliance again.