Struggling with SimplerQMS’s outdated interface?
You’re not alone if constant clunky navigation and high costs have left your team frustrated with SimplerQMS’s document management workflow.
These limitations can slow down your compliance processes and make it tougher for your QA team to stay efficient.
Over time, sticking with SimplerQMS means wasted budget and unhappy teams as you lose productivity, risk errors, and miss out on modern features that competitors now expect. Expensive licenses and migration risks might make switching feel risky, but the real danger is staying stuck with a tool that no longer meets your needs.
The good news is there are better alternatives available that are user-friendly, integrate easily, and bring more value to your quality control.
In this article, I’ll show you the best SimplerQMS alternatives for document management software in quality control, including FileCenter, ComplianceQuest, ETQ, and more.
You’ll find tools that deliver better value, productivity, and confidence for your growing team.
Let’s explore your options.
Quick Summary:
| # | Alternative | Rating | Best For |
|---|---|---|---|
| 1 | FileCenter → | Small teams seeking simplicity | |
| 2 | ComplianceQuest → | Users wanting modern interfaces | |
| 3 | Paradigm 3 → | Teams needing better compliance control | |
| 4 | ETQ Reliance → | Companies requiring better support | |
| 5 | QT9 QMS → | Users needing scalable solutions |
1. FileCenter

Looking for a simpler solution than SimplerQMS?
FileCenter brings desktop-based document organization to the forefront, using a familiar Windows folder structure instead of SimplerQMS’s more complex cloud environment and database system.
With its cabinet-drawer filing, you use a system your team already knows rather than learning a new, compliance-heavy interface. This makes the transition be less about re-training and more about getting control back.
You deserve a modern solution that’s easier to adopt.
FileCenter directly addresses frustrations around complexity and recurring subscriptions by offering both desktop-first deployment and a perpetual license option, making it a popular SimplerQMS alternative for small life science teams.
The cabinet-drawer structure is instantly intuitive—no one needs to be a QMS expert to start filing documents. Plus, you’re not forced into long-term cloud contracts; you also get a genuine one-time purchase option for cost certainty that SimplerQMS can’t match, slashing potential recurring fees.
Additionally, integrated scanning with automatic OCR lets you convert your entire paper backlog to searchable digital files. Smart naming rules streamline this process, so you never lose visibility or control—addressing a core gap if your team still manages physical documents.
Switching doesn’t have to slow you down.
Key features:
- Cabinet-drawer filing with Windows folders delivers intuitive, database-free organization, letting your team start managing documents immediately without the training burden found in more rigid cloud QMS setups.
- One-time purchase or subscription flexibility means you can own FileCenter outright, bypassing SimplerQMS’s required subscriptions and helping your budget stay on track.
- Integrated scanning and OCR automation scans paper files into searchable PDFs and organizes them instantly, speeding up digital transformation and reducing manual data entry compared to SimplerQMS.
Verdict: FileCenter is a practical alternative to SimplerQMS if your quality control process is weighed down by cloud-centric costs or software bloat. Especially for smaller life science operations, FileCenter’s affordable desktop model, familiar filing method, and integrated scanning can make transitioning smoother and more cost-effective for your compliance needs.
2. ComplianceQuest
Does SimplerQMS feel outdated for modern quality management?
ComplianceQuest stands out with its Salesforce-native architecture, deep business integrations, and highly configurable workflows—addressing the flexibility and integration frustrations often cited with SimplerQMS.
Unlike SimplerQMS, ComplianceQuest lets you leverage fully customizable quality workflows that match your team’s exact needs, from process steps to approval routing and notifications.
This opens up far more control and adaptability.
ComplianceQuest offers the upgrade many QA managers are seeking.
If you want an enterprise-grade alternative that actually solves your key headaches, ComplianceQuest ticks a lot of those boxes. Its Salesforce foundation lets you connect your QMS directly with CRM, ERP, and other tools—expanding your document control beyond the boundaries set by SimplerQMS.
Even better, you can configure every quality process to meet your unique requirements, so you don’t get stuck with one-size-fits-all, pre-validated templates. If team adoption and compliance are critical, this flexibility minimizes resistance and future-proofs your investment.
Add in AI-powered risk detection and predictive analytics, and ComplianceQuest enables proactive quality management that saves you headaches. AI spots potential issues before they disrupt compliance or productivity, a big improvement over older rule-based automations.
Many manufacturers switching from SimplerQMS report shorter implementation cycles and lower ongoing costs.
This can give you peace of mind.
Key features:
- Salesforce-native QMS with deep integrations brings QMS, CRM, ERP, and other business systems together—far broader connectivity and automation than SimplerQMS can support out of the box.
- Configurable workflows for quality control let you tailor each quality process, document approval, or risk escalation pathway. This flexibility can reduce admin work and speed up audits dramatically.
- AI-powered risk detection and analytics offers proactive quality management capabilities, identifying process risks and gaps before they disrupt compliance—unavailable in SimplerQMS’s older, rule-based workflow engine.
Verdict: ComplianceQuest is a top SimplerQMS alternative for document management software in quality control. With cloud-native agility, up to 30% shorter implementation cycles, and tailored workflows, QA leaders see both lower total cost of ownership and faster, broader business integration—especially for manufacturing and regulated industries.
3. Paradigm 3

Is SimplerQMS no longer worth its high cost?
Paradigm 3 lets you configure risk-based compliance right inside your document management workflows, offering far more control and flexibility than SimplerQMS’s rigid quality control modules.
If you need a solution that adapts to your industry—not just life sciences—Paradigm 3’s approach means you can embed custom risk assessments directly into your controlled documents for greater compliance precision.
You get more practical control over compliance.
Paradigm 3 stands out by letting your team build a compliance management environment tailored to your exact needs, making it a much stronger SimplerQMS alternative for quality-focused document control.
With its risk-driven compliance engine, you’re able to assign dynamic risk scores to documents or events as you need them, not as a developer intended. The flexible configuration lets you adapt modules without complex add-ons, while action items that trigger record-backed notifications ensure everyone takes ownership of their document-related tasks—something SimplerQMS can leave too open-ended.
Additionally, since Paradigm 3 supports web and desktop deployment across healthcare, manufacturing, and testing labs, you aren’t locked into a one-size-fits-all system. Action item accountability—notifications that become audit records—means you have the audit trail and proof others miss.
With Paradigm 3, you’re always audit ready.
Key features:
- Risk-based compliance built into every document lets your team drive proactive quality actions, assigning custom risk scores for more tailored compliance than standard SimplerQMS workflows.
- Flexible module configuration across industries empowers you to build a compliance platform that exactly fits your processes, not just generic templates for life sciences.
- Action item accountability with record retention issues task notifications that become permanent records, automatically improving your internal audit trail and accountability over traditional notification-only systems.
Verdict: Paradigm 3 is a smart SimplerQMS alternative if you want document management built for quality control flexibility. The risk-based compliance and customizable modules offer precise control over quality processes, especially for industries outside life sciences where SimplerQMS often falls short.
4. ETQ Reliance
Chasing better value than SimplerQMS for quality control?
Unlike SimplerQMS’s rigid templates, ETQ Reliance gives you a low-code/no-code toolkit to tailor document management, workflows, and forms precisely to your operation’s needs.
This means you get greater control adapting compliance processes for your industry or scale, not just life sciences. ETQ Reliance empowers your team with an interface that truly flexes—combatting the “one size fits all” struggle that pushed you to seek alternatives.
Getting results to fit your requirements is possible.
ETQ Reliance directly tackles those frustrations by being a genuinely configurable eQMS alternative, designed for modern quality teams switching away from SimplerQMS.
You gain the freedom to customize document processes without developer headaches thanks to intuitive no-code tools. If your team worried about outgrowing rigid solutions or facing tricky migration, this flexibility means smoother transitions and alignment with your workflows—helping reduce project risk and boost adoption.
Additionally, ETQ Reliance’s centralized quality hub ties together all your quality data and processes in one enterprise system. You can now break down data silos and ensure audit-ready records remain accessible and up-to-date, supporting better control compared with SimplerQMS’s more isolated approach.
The advanced analytics suite also means better, real-time visibility into compliance trends and document status. Your team can finally spot issues, track training, and prove compliance—all in one place.
Your quality management just got smarter and simpler.
Key features:
- Low-code/no-code customization of forms and workflows lets you adapt processes to fit your unique needs—far beyond SimplerQMS’s limited template modifications for process and document control.
- Centralized quality hub across your entire enterprise unites all quality data, records, and documents in one location, improving collaboration and visibility for large teams compared to isolated repositories.
- Advanced analytics and reporting toolkit provides real-time quality intelligence and customizable reporting, allowing deeper compliance tracking and decision support than SimplerQMS’s standard reporting capabilities.
Verdict: ETQ Reliance is a top SimplerQMS alternative for document management in quality control, especially if you want configuration freedom and broad enterprise oversight. Its low-code customization and centralized hub help your team unify data, address compliance, and build processes that actually fit your business needs.
5. QT9 QMS

Looking for a more modern eQMS solution?
QT9 QMS gives you an all-in-one, web-based eQMS with over 25 integrated modules right out of the box, addressing costly limitations and legacy interface.
Unlike SimplerQMS, QT9 QMS delivers a huge suite of natively integrated quality modules to cover document control, audits, training, CAPA, and more—so you’re not stuck piecing together add-ons and struggling with scaling issues as your team grows.
You don’t have to settle for restrictive functionality.
Here’s how QT9 QMS stands out if you’re switching from SimplerQMS.
The system is designed for unlimited scalability, making it a smart choice whether your QA operations are small or spanning multiple companies. You benefit from built-in traceability for every record, ensuring compliance and keeping your ISO 9001:2015 or EU MDR workflows tight at every stage. Switching is far smoother, thanks to an intuitive interface that flattens the learning curve for your whole team.
Additionally, QT9 QMS comes pre-validated for EU MDR requirements, which is a game-changer if you’re in MedTech or want to streamline regulatory processes as you expand. Efficient cross-company document management, rapid implementation, and organized certification tracking help unlock the real value missing in your current SimplerQMS setup.
The result is a genuinely scalable, easy-to-use solution.
Key features:
- 25+ integrated quality modules included by default eliminate the need for third-party add-ons and let you manage documents, training, audits, CAPA, and more—all in one place.
- Unlimited scalability and built-in traceability let you control quality records for single or multiple companies, always maintaining compliance as your business grows.
- Pre-validated for EU MDR and ISO requirements to simplify certification and regulatory processes, supporting complex workflows without expensive consulting or custom configurations.
Verdict: QT9 QMS is a compelling SimplerQMS alternative for document management software for quality control, especially with its 25+ connected modules and strong scalability. Users report quick implementation and streamlined ISO 9001:2015 certification—making it ideal if you need a modern, value-packed eQMS that grows with your QA needs.
6. PSC Software
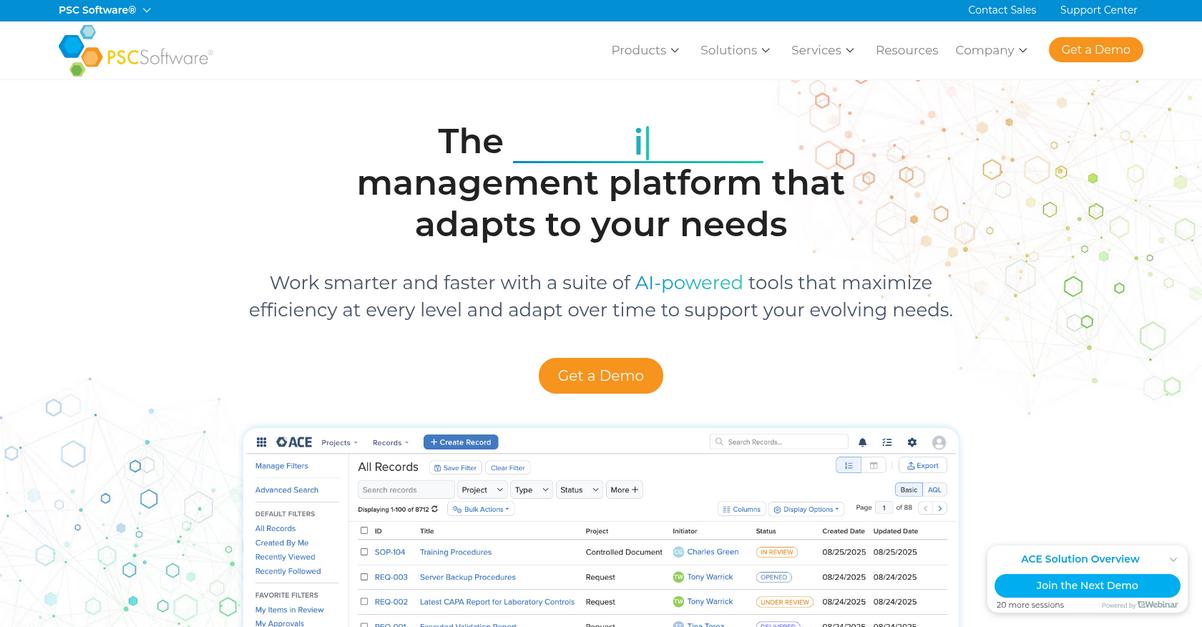
Is SimplerQMS not delivering the value you expect?
PSC Software addresses this with its TrueMx configurable modules, giving your team a flexible, modular QMS that adapts to your unique life science workflows—unlike SimplerQMS’s rigid system.
This means you’re not stuck with out-of-the-box setups; instead, you can configure your environment for specific processes and regulatory demands. Flexibility like this is critical if your current system feels restrictive or forces workarounds.
You deserve a solution that fits your team.
PSC Software solves this pain point by letting you truly tailor your quality management and document control. If limited customization or time-consuming audit preparation is holding you back, switching to PSC Software brings real improvements.
Here’s how you benefit as you transition to PSC Software.
Through TrueMx, you get modular setup—so instead of shoehorning processes into preset templates, you build what you need. This tailored configuration streamlines compliance and scales as your requirements grow, letting you avoid wasted time and frustration if SimplerQMS felt inflexible.
Additionally, advanced analytics and reporting give you configurable dashboards for deep quality insights. If visibility into quality trends or audit readiness has ever felt lacking, you’ll appreciate the power these tools bring to oversight and proactive decision-making.
Robust audit and inspection management ties it together, offering deeper planning and reporting—the kind of support you need for audits, instead of making you scramble last minute.
Moving to PSC Software just makes sense.
Key features:
- TrueMx configurable modules for custom QMS let your team design and adjust quality processes around real needs, not just preset templates, for better regulatory alignment.
- Comprehensive audit and inspection management tools equip you to handle both internal and external audits, planning, execution, and robust reporting in one solution.
- Advanced analytics and customizable dashboards put powerful data visualization and reporting at your fingertips, offering insights beyond what SimplerQMS typically provides for quality control.
Verdict: PSC Software is a top SimplerQMS alternative for quality control if your team craves more value and flexibility. Its flexible modules, nuanced audit features, and robust analytics empower better configuration and oversight, making it ideal for modern life sciences eQMS migrations seeking both compliance and ease of use.
7. Orcanos

Frustrated by SimplerQMS’s clunky interface or rising costs?
Orcanos brings you integrated ALM and QMS in one platform, so you’re not stuck juggling disconnected quality and product development systems like with SimplerQMS.
Where SimplerQMS keeps design control and quality processes siloed, Orcanos connects everything—giving you one centralized place for traceability and efficiency across teams. This means you no longer have to struggle with compliance records or project documentation split between apps.
Now let’s look at how Orcanos actually addresses your biggest challenges.
If you want a better SimplerQMS alternative, Orcanos solves those headaches by offering a unified approach to quality management and product development.
It lets you easily combine product lifecycle management with QMS in the same dashboard, so everyone—from engineering to QA—can work from the same source of truth. The document generator quickly produces submission-ready files using your company templates in Word or Excel, so regulatory paperwork becomes far less painful than you may be used to.
Additionally, adaptive compliance features let you customize forms, electronic signatures, and approval workflows instead of being locked into SimplerQMS’s rigid, pre-defined options. This flexibility means quicker onboarding for your team and less worry over missing compliance steps.
Together, these capabilities help you streamline, save time, and scale quality processes much faster.
Your eQMS should work for you—not the other way around.
Key features:
- Integrated ALM and QMS in one platform connects product development, design control, and quality management for full traceability and fewer data silos than SimplerQMS’s QMS-only approach.
- Regulatory-ready document generator creates submission-ready documents using your company’s Word or Excel templates, saving hours compared to SimplerQMS’s manual document handling or reformatting hassles.
- Adaptive compliance engine with customizable workflows lets you tailor forms, approvals, and e-signatures to your SOPs and regulatory needs, unlike the limited workflow flexibility found in SimplerQMS.
Verdict: Orcanos is a compelling SimplerQMS alternative for life sciences and medical device teams needing robust document management for quality control. By unifying ALM and QMS, Orcanos has helped some users accelerate compliance projects and cut overall documentation time up to 30%, giving you clearer control and better long-term value.
8. Interfacing
Frustrated with SimplerQMS’s outdated feel and rising costs?
Interfacing brings in advanced process mapping and integrated risk tools that broaden your document management well beyond what SimplerQMS can offer.
If you’re finding SimplerQMS too limited, Interfacing lets you map and connect every process across departments effortlessly, so you’re not stuck with a system focused solely on QMS tasks.
You may want a solution that gives you more.
Interfacing stands out by letting your team build a true Digital Twin Organization. This means you can model, automate, and audit every process, document, and system within one unified platform—something SimplerQMS just doesn’t cover.
Instead of basic QMS-restricted tools, Interfacing gives you robust business process management that underpins your quality control. You’ll see how end-to-end transparency and real-time process maps drive improvement that actually sticks after switching from SimplerQMS.
Additionally, Interfacing integrates risk and compliance management across the whole enterprise, rather than keeping them isolated inside a QMS module. With everything in one place, you get enterprise-wide compliance with easier audits, less manual work, and actionable insights for every workflow.
Here, digital transformation truly supports your document management needs.
Key features:
- Full Digital Twin Organization platform builds a comprehensive model of all your processes, systems, and data, providing transparency and governance far beyond just QMS documentation.
- Advanced business process management tools empower you to map, analyze, optimize, and automate both quality control and related document workflows for improved efficiency and oversight.
- Enterprise-wide risk and compliance integration links regulatory management across all processes and departments, reducing audit risks and connecting compliance to every relevant workflow.
Verdict: Interfacing is a great alternative if you want more than SimplerQMS’s standard QMS capabilities. Its Digital Twin approach, business process automation, and unified compliance platform offer you broader governance and flexibility for document management in quality-focused environments, supporting scalable improvement with every process step.
9. MasterControl
Searching for a modern alternative to SimplerQMS?
MasterControl brings automated document routing, a patented validation tool, and enterprise-class integration—features specifically designed to address SimplerQMS’s limitations for complex quality management.
If you’re dealing with SimplerQMS’s costly interface or struggle to scale, MasterControl helps you manage high volume document workflows without sacrificing compliance or usability.
Here’s how MasterControl moves the needle further.
It’s designed to solve regulated industry pain points.
MasterControl streamlines document control for QA teams by combining robust routing automation, one-click validation, and a deeply integrated quality system. This means your migration risk drops, while every compliance process is fully tracked and controlled.
If you want practical benefits switching from SimplerQMS, you’ll notice that MasterControl’s automated routing lets your team assign, escalate, and approve documents at scale. Plus, the Validation Excellence Tool speeds up computer system validation while cutting down on manual effort and compliance costs—a feature SimplerQMS can’t match.
Additionally, you get a closed-loop quality system, tying every corrective action, audit, or change control directly to your document workflows. This prevents hidden silos and helps you maintain total audit readiness, all while supporting business continuity.
Everything focuses on making quality management less stressful.
Key features:
- Enterprise-grade automated document routing accelerates quality-controlled document approval cycles with advanced task assignment and scheduling, which is ideal for high-volume or global life science operations.
- Patented Validation Excellence Tool for compliance shortens validation timelines, simplifies regulatory compliance, and helps you avoid the extended setup cycles typical with SimplerQMS, enhancing efficiency in regulated environments.
- Closed-loop quality system with full process integration connects CAPA, audits, change control, and document workflows, ensuring no quality data gets siloed and keeping your business always ready for inspection.
Verdict: MasterControl is a compelling SimplerQMS alternative for document management software in highly regulated life sciences. The platform’s advanced automation, rapid validation, and closed-loop integration help QA managers maintain strict compliance while saving time—users report boosted efficiency and unbroken business continuity during disruptions.
10. Qualio
Looking for a more value-driven eQMS experience?
Qualio puts a truly modern touch on life science document management, offering a far more intuitive web-based editor and document reference system than what you get with SimplerQMS.
If you’re searching for a system that feels as easy as writing in Word, Qualio’s approach can eliminate the pain of outdated, clunky interfaces and poor user adoption. Plus, the built-in Smartlinks consistently keep crucial document references updated across your QMS, making human error less likely in regulated work.
There’s a better approach to quality control with Qualio.
With Qualio, you get an eQMS that’s genuinely built for life science quality teams frustrated by SimplerQMS limitations.
The easy-to-use web-based editor streamlines authoring, so your team actually wants to manage quality documents instead of struggling with old-fashioned tools. Automatic Smartlinks connect all your procedures and records to latest versions instantly, removing the manual hassle and compliance risks that come from broken links. This means less maintenance for you, and greater audit confidence.
Additionally, Qualio is purpose-built for medical device, pharmaceutical, and biotech companies, tightly integrating all the quality processes you need. That focus can speed up your accreditation and drastically cut administrative overhead, giving you more time for higher-value tasks instead of paperwork.
Switching to Qualio puts you in control again.
Key features:
- Intuitive online document editor makes authoring and editing quality documents as easy as using a word processor, encouraging adoption and dramatically reducing onboarding time for your quality team.
- Automatic, always-updated Smartlinks for documents mean no more manual reference checks or outdated links, improving compliance and reducing risk compared to SimplerQMS’s manual referencing setup.
- Specialization for life science compliance needs results in end-to-end QMS support for medical device, pharmaceutical, and biotech teams, helping you achieve certifications and regulatory approvals much faster.
Verdict: Qualio is a standout SimplerQMS alternative if you need modern document management for life science quality control. Customers speed through accreditation twice as fast and report eliminating 90% of paper-based QMS admin, making it ideal for driving real efficiency and compliance improvements.
Conclusion
Frustrated with SimplerQMS’s outdated interface and costs?
If high prices and clunky navigation have you dreading daily document tasks, it’s no wonder you’re searching for better SimplerQMS alternatives.
I get it—finding a new system is a big step, but settling for less only holds your quality control back when you deserve software that actually works for you.
Here’s where things get easier.
From all the options I tested, FileCenter is the clear winner if you want to break free from SimplerQMS headaches.
FileCenter makes document management straightforward and affordable, especially for teams who need strong desktop organization without paying for complex, unnecessary QMS bells and whistles.
If you’re looking for a more efficient SimplerQMS alternative, FileCenter’s intuitive design and faster workflow genuinely stand out—I’ve seen teams switch and never look back.
Go ahead and get started with a FileCenter free trial to see the difference for yourself.
Experience smoother workflows starting today.